Analysis of an Active Deformylation Mechanism of 5‐Formyl‐deoxycytidine (fdC) in Stem Cells
2020-01-30
M. Sc. Alexander Schön, Ewelina Kaminska, M. Sc. Florian Schelter, M. Sc. Eveliina Ponkkonen, M. Sc. Eva Korytiaková, Dr. Sarah Schiffers, Thomas Carell
Angew. Chem. Int. Ed., 2020, 59 (14), 5591–5594
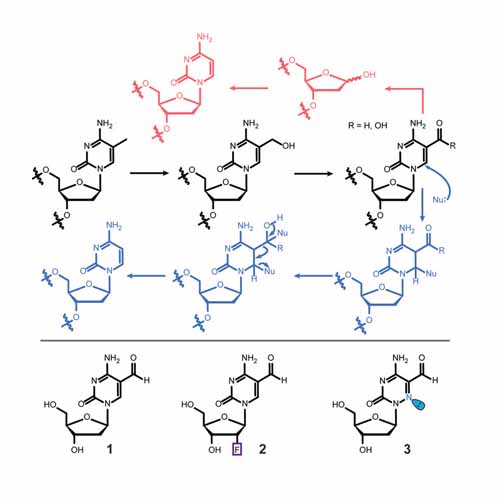
The removal of 5‐methyl‐deoxycytidine (mdC) from promoter elements is associated with reactivation of the silenced corresponding genes. It takes place through an active demethylation process involving the oxidation of mdC to 5‐hydroxymethyl‐deoxycytidine (hmdC) and further on to 5‐formyl‐deoxycytidine (fdC) and 5‐carboxy‐deoxycytidine (cadC) with the help of α‐ketoglutarate‐dependent Tet oxygenases. The next step can occur through the action of a glycosylase (TDG), which cleaves fdC out of the genome for replacement by dC. A second pathway is proposed to involve C−C bond cleavage that converts fdC directly into dC. A 6‐aza‐5‐formyl‐deoxycytidine (a‐fdC) probe molecule was synthesized and fed to various somatic cell lines and induced mouse embryonic stem cells, together with a 2′‐fluorinated fdC analogue (F‐fdC). While deformylation of F‐fdC was clearly observed in vivo, it did not occur with a‐fdC, thus suggesting that the C−C bond‐cleaving deformylation is initiated by nucleophilic activation.